Differential Analysis Continue Cups Alt 1 or Discontinue for the Month Ended May 31
Continuing Education Activity
Open-angle glaucoma is a chronic, progressive, and irreversible multifactorial optic neuropathy that is characterized by an open angle of the anterior chamber, optic nerve head changes, progressive loss of peripheral vision, followed by central visual field loss. Elevated intraocular pressure is an important risk factor for open-angle glaucoma and can be a result of primary or secondary causes. Improved knowledge of open-angle glaucoma will allow for the prevention of devastating blindness caused by untreated open-angle glaucoma. This condition is asymptomatic and will slowly and irreversibly erode a patients eyesight. This activity outlines the evaluation and management of open-angle glaucoma and highlights the role of the interprofessional interprofessional team in managing patients with open-angle glaucoma.
Objectives:
-
Identify the risk factors for open-angle glaucoma.
-
Explain how to diagnose open-angle glaucoma.
-
Summarize the treatment options available for patients with open-angle glaucoma.
-
Review strategies for improving care coordination and communication amongst interprofessional teams to advance treatment of open-angle glaucoma and improve patient outcomes.
Access free multiple choice questions on this topic.
Introduction
Glaucoma refers to a collection of diseases whereby increased intraocular pressure adversely impacts the optic nerve, and subsequently, the visual field. However, not all cases of glaucoma are associated with increased intraocular pressure, a subset includes similar optic nerve damage and visual field damage known as normal pressure glaucoma. The collection of glaucomatous diseases is subdivided into open-angle and closed-angle glaucoma, both of which can have primary or secondary causes. Open-angle glaucoma (OAG) is a chronic, progressive, and irreversible multifactorial optic neuropathy that is characterized by open angle of the anterior chamber, typical optic nerve head changes, progressive loss of peripheral vision (typical visual field changes) followed by central visual field loss (blindness) for which intraocular pressure (IOP) is an important risk factor. The disease is usually bilateral, but asymmetry is often seen depending on the etiology. It is important to note that increased intraocular pressure is not a necessary prerequisite for glaucomatous optic nerve damage, nor are the two necessarily correlated as studies have shown individuals witch chronically elevated intraocular pressures being asymptomatic.
Etiology
In order to understand the etiology of open-angle glaucoma, it is important to understand the formation and drainage of aqueous humor. Aqueous humor is produced in a continuous fashion by the ciliary body in the posterior chamber and drains into the anterior chamber of the eye. A majority of aqueous humor drainage occurs through the trabecular meshwork and a minority of aqueous outflow is drained through the uveoscleral pathway. Primary open-angle glaucoma is the most common type of glaucoma and is characterized by increased resistance to drainage in the trabecular meshwork, even though the drainage angle between the cornea and iris remains open. Due to this blockage, the pressure in the eye gradually increases, resulting in damage to the optic nerve and progressive visual loss. Secondary open-angle glaucoma can have multiple etiologies but is far less common than primary open-angle glaucoma.
Genetic Etiologies
Chromosome 1q24.3, MYOC gene: Myocilin is a cytoskeletal protein product of the MYOC gene, which is implicated in cases of hereditary juvenile OAG and hereditary adult OAG. Myocilin is a cytoskeletal protein expressed in the trabecular meshwork and is also known as trabecular meshwork glucocorticoid-inducible response protein (TIGR).[1]
Chromosome 5q22.1, WDR36 gene: The WDR36 protein is a member of the WD repeat protein family, and is thought to be implicated in adult-onset POAG. This protein functions in ribosomal RNA processing, p53 stress-pathway response, cell cycle progression, signal transduction, apoptosis, and gene regulation. WDR36 protein is expressed in the lens, iris, sclera, ciliary muscles, ciliary body, trabecular meshwork, retina, and optic nerve in the eye but is also expressed outside of the eye in the human heart, placenta, liver, skeletal muscle, kidney and pancreas. Four mutations in WDR36 at the GLC1G locus (N355S, A449T, R529Q, and D658G) have been identified, with one study implicating the gene in approximately 6% of POAG patients. However, a recent 2017 Chinese study repudiated this claim by showing that the association between WDR36 and POAG is not consistent across populations and calls for more data supporting WDR36 protein's role in POAG. [2][3]
Chromosome 7q31, CAV1/CAV2 genes: CAV1 and CAV2 are proteins members of the caveolin family that are involved in the formation of caveolae- invaginations of the plasma membrane in areas that are rich in cholesterol during the process of transcytosis. This gene is associated with POAG susceptibility in populations of European and East Asian ancestry.CAV1 and CAV2 are expressed in most human cell types, including tissues such as the scleral spur cells, trabecular meshwork, and retinal ganglion cells. In vitro studies of CAV1 showed consistent upregulation in the trabecular meshwork after one hour of increased IOP.[4][5][4]
Chromosome 9p21, CDKN2B-AS1 gene: CDKN2B-AS1 is a cyclin-dependent kinase inhibitor 2B antisense noncoding RNA that regulates cyclin-dependent kinase inhibitor 2A and 2B in the cell cycle. US-based observational case study, we found that this region modifies optic nerve vulnerability to glaucomatous change. It is thought that an SNP in this gene is implicated in POAG by causing RGCs to undergo apoptosis during their quiescent post-mitotic state. [6][7][8]
Chromosome 10p13, OPTN gene: Optineurin the coiled-coil protein product that is implicated in adult-onset primary open-angle glaucoma and in normal-tension glaucoma. Optineurin is involved in various cellular functions including apoptosis, cellular morphogenesis, inflammation, vasoconstriction, membrane protein trafficking, vesicular trafficking as well as transcription activation. [9]
Chromosome 15q24.1, LOXL1 gene: Lysyl oxidase-like 1 gene codes for an extracellular copper-dependent amine oxidase enzyme that catalyzes the first step in crosslinking of collagen and elastin in the extracellular matrix and is implicated in cases of pseudoexfoliation syndrome. Single nucleotide polymorphism in the LOXL1 gene is associated with excessive levels of crosslinked amyloid-like fibrillar glycoproteins that deposit in the anterior segment and is more common in Scandinavian populations. SNPs in LOXL1 gene can present as exfoliation glaucoma as the first signs of a more serious systemic condition that implicates multiple tissues with the expression of this enzyme including the liver, kidney, and gallbladder.[10][11][12][13]
Epidemiology
Glaucoma affects 70 million people worldwide and is the second leading cause of blindness both in the United States and across the entire planet. Of these 70 million glaucoma sufferers worldwide, 74% suffer specifically from open-angle glaucoma and in the United States, nearly 80% of all glaucoma is OAG. Nearly 10% of all glaucoma patients are bilaterally blind, and bilateral blindness due to OAG has been estimated to affect 5.9 million people in 2020. There are at least 2.7 million people aged ≥40 years with glaucoma in the United States, and the number of patients suffering from all forms of glaucoma worldwide is projected to rise to 79.6 million by 2020. The highest prevalence of OAG can be found in African-American patient populations (Glaucoma is the leading cause of blindness in African-Americans) with Latin American and Chinese prevalence approaching African-American levels in older patients populations. Women are disproportionately affected by all forms of glaucoma, comprising approximately of 55.4% of all cases of OAG. It is important to remember that longevity also plays a role because glaucoma affects primarily elder populations. The longevity factor should be taken into consideration when considering the epidemiology of glaucoma as both women and developed countries have greater longevity compared to male and non-developed countries, respectively. By 2020, OAG is projected to have the highest prevalence rate in Europe followed by China and India.[14][15]
Risk Factors:
-
Old age (African American over 40 and Caucasian over 65 years old) [16]
-
Race (African-American, Afro-Caribbean, and West African patients have a four-fold increased risk of developing OAG) [16]
-
Family history (The Rotterdam Eye study found 9.2 times higher risk of developing OAG if first degree relatives had glaucoma) [17]
-
Elevated IOP
-
Myopia
-
Increased cup-to-disc ratio
-
Disc hemorrhage
-
Thin central corneal thickness
-
Low ocular perfusion pressure
-
Low blood pressure (systolic and diastolic)[18]
-
High blood pressure (systemic arterial hypertension has been associated but not confirmed risk factor for OAG)[19]
-
Type 2 diabetes mellitus
-
High pattern standard deviation on visual fields
-
Migraine or vasospasm
-
Low intracranial (CSF) pressure
-
Oral contraceptive pill
-
Other risk factors include smoking, obesity, alcohol, anxiety, stress, and sleep apnea
Pathophysiology
Retinal Ganglion cells
It is important to appreciate that the exact cause of glaucoma is not fully known, but the underlying pathology lies in the apoptosis of retinal ganglion cells. Retinal ganglion cells (RGC) are the third class of photoreceptors that have recently been noted in the scientific literature for their intrinsic photosensitivity. RGCs form functional microcircuits with rods, cones, amacrine cells, and bipolar cells that help transmit both image forming and non-image forming information to the brain. RGC axons target the suprachiasmatic nucleus (SCN), intergeniculate leaflet (IGL), and olivary pretectal nucleus (OPN), ventral division of the lateral geniculate nucleus (LGv), and the preoptic area. RGCs have been found to play a role in modulating circadian rhythm and the release of melatonin, regulating pupil size, and forming an important relay in on-off centers of the retina. It is also important to note that RGC axons remain unmyelinated until they have passed through the lamina cribrosa and have converged to form the optic nerve (cranial nerve 2). Only after passing through the lamina cribrosa do we see oligodendrocytes myelinating the axons of the RGCs. Iatrogenically induced ocular hypertension in mice, cats, and monkeys have shown blockade of both orthograde and retrograde axonal transport, which also plays an integral role in transporting growth factors such as brain-derived neurotrophic factor. Regardless of whether intraocular pressure is elevated, normal, or decreased- it is the death of RGCs that underlies the pathology of glaucoma. [1][20][21][22]
Lamina Cribrosa
The lamina cribrosa is a sieve-like fenestration at the back of the sclera that allows for a conglomeration of retinal axons and blood vessels to exit the posterior of the eye. Changes in the three-dimensional structure of the lamina cribrosa have been implicated in the pathogenesis of glaucomatous optic atrophy. The superior and inferior channels of the lamina cribrosa contain larger pores and thinner connective tissue support for the passage of nerve-fiber bundles than the nasal and temporal parts of the lamina. It is interesting to note that the most common pattern of glaucomatous optic nerve thinning occurs at the inferior and superior portions of the optic nerve. Also, the superior and inferior laminar zones of the lamina cribrosa is where the arcuate area ganglion cell axons travel through and these axons are the most susceptible to glaucomatous damage. It is thought that mechanical damage to axons and the prevention of essential trophic factors, such as brain-derived neurotrophic factor (BDNF) being appropriately delivered is conducive to the disease process. Studies have found that statistically significant depth variability exists among the superior and inferior lamina cribrosa of healthy patients and those with OAG. Patients suffering from OAG were found to have greater depth at the optic cup floor, possibly as a result of increased IOP pressure. One study looked at the lamina cribrosa using Heidelberg Retina Tomograph and found a greater level of topographic variability and "spikiness" when looking at the lamina cribrosa of OAG patients. This is most likely a sign of fragility in the lamina, as increased spikiness was inversely related to Humphrey mean deviation (P<0.05), and cup-disc ratio (P<0.004) and was directly related to nerve fiber layer thickness (P<0.005). [23][24][25]
Elevated Intraocular Pressure
High IOP is an important risk factor for the progression of glaucoma. Of the risk factors causing open-angle glaucoma, the most studied risk factor had been elevated IOP, as it is modifiable. It has been shown that once IOP rises above 21 mmHg, there is a significant increase in the risk of developing visual field loss (even with only small increases in IOP), especially once IOP rises above 26 mmHg to 30 mmHg. The high fluctuation of IOP may also lead to glaucoma progression. Reduction of IOP leads to less progression or stabilization of the glaucomatous optic nerve changes and visual field changes. About 40-50% of all OAG cases have IOP below 22 mm Hg in a single screening.[26][19]
Aqueous Outflow
The two main proposed mechanisms by which an elevated IOP is thought to contribute to glaucomatous damage includes vascular dysfunction resulting in ischemia to the optic nerve, and mechanical dysfunction as a result of compression of the axons. When open-angle glaucoma in a patient is attributed to elevated IOP, the cause of this increase in IOP is commonly thought occur due to dysfunction in aqueous outflow through the trabecular meshwork of the eye. This may occur as a result of partial obstruction due to foreign material (e.g., accumulated mucopolysaccharides, in the trabecular meshwork), a reduction in the number of trabecular endothelial cells, a decreased density of trabecular pores, number of vacuoles, or size of the inner wall endothelium of the canal of Schlemm, loss of phagocytic activity, or dysfunction in the neurological feedback loop involved in drainage of aqueous humor. Other proposed mechanisms for obstruction of aqueous humor outflow include oxidative damage to the meshwork, abnormal corticosteroid metabolism, adrenergic dysfunction, or an immunological process. It is important to note that unlike angle-closure glaucoma, the drainage angle between the iris and cornea remain open in open-angle glaucoma. Finally, it has been proposed that certain individuals may have a genetic predisposition to cell death of individual axons in the eye, resulting in the release of potentially cytotoxic substances such as glutamate, calcium, nitric oxide, and free radicals, as well as apoptosis of neighboring cells.
Histopathology
Early in the disease process, glaucomatous optic nerve atrophy will present with thinning and atrophy of the retinal ganglion cell layer and thus, thinning of the nerve fiber layer above the ganglion cells. The nerve fiber layer consists of the unmyelinated ganglion cell axons, hence they shrink concomitantly. In more advanced glaucomatous optic nerve atrophy there will be both cupping of the optic nerve as well as atrophy of the ganglion cell layer and subsequently the nerve fiber layer. As the ganglion cells deteriorate the structural integrity of the nerve is compromised. Hence, increased intraocular pressure will push into the optic nerve and cause the visible phenomenon of cupping. Retinal ganglion cell loss also leads to increased space and widening of the subdural optic nerve space. [27]
Toxicokinetics
In open-angle glaucoma, particulate matter can lodge into the fine openings in the trabecular meshwork and thus increase the passive resistance to aqueous humor drainage.
Lens proteins: During cataract surgery, high-molecular-weight lens proteins produced as a by-product of phacolysis can lodge into the trabecular meshwork and increase drainage resistance.[28][29]
Red blood cells: In the event of a traumatic injury to the eye, senescent red blood cells can become lodged in the trabecular meshwork- a variant of open-angle glaucoma known as "Ghost cell glaucoma."[30]
Pigment granules: pigment from the epithelium of the iris can detach and become lodged in the fine trabecular meshwork, pigmentary glaucoma, and pigment dispersion syndrome.[28]
Tumor debris: Necrotic tumor debris from necrotic tumors can also clog the trabecular meshwork in a variant known as melanomalytic glaucoma.[31]
Corticosteroids: Steroid therapy of any kind can contribute to elevated intraocular pressure, however, topical eye and periocular steroids a most likely to increase intraocular pressure. [16]
History and Physical
Open-angle glaucoma is often asymptomatic in its early stages, therefore, a thorough and comprehensive history and exam can be instrumental in detecting the disease early. Early changes in OAG involves a loss of peripheral vision that the patient is usually not aware of until 40% of their nerve fibers have been compromised, only then do they start to notice having "tunnel vision." Elderly patients might give clues in their history to their loss of peripheral vision by admitting to difficulties driving or running into objects around the home more frequently. In a primary care setting, patients with the aforementioned risk factors should undergo direct ophthalmoscopy in order to visualize the optic disc. Visualization of the optic disc can provide reliable diagnostic information as it often shows changes before the visual field deficits are symptomatic. OAG is often bilateral and the two optic discs can be compared to one another as a useful metric, but the damage can also be asymmetric as well. Primary care physicians should refer patients with the aforementioned risk factors, clinical findings, or symptoms suggestive of glaucoma to an ophthalmologist.
-
A previous ocular history such as the history of eye pain or redness, headaches, uveitis, diabetic retinopathy, cataracts, vascular occlusions, or multicolored halos
-
African-American Race
-
Refractive error
-
Chronic use of topical or systemic corticosteroids
-
Ocular surgery like photocoagulation or refractive procedures, cataract surgery, glaucoma surgery, and systemic surgery/ medications
-
Head or ocular trauma
-
Take certain medications such as hypertensive medications that may influence IOP or systemic/topical steroids
-
Medical history of diabetes mellitus, migraine headaches, hypertension, vasospasm, cardiovascular disease, breathlessness, cardiac arrhythmia
-
Family history (e.g., first-degree relative with glaucoma, especially significant if this is a sibling) that would place them at a greater risk of developing open-angle glaucoma.
-
Old medical documentation of IOP, optic disc, visual field, and others.
Evaluation
Open-angle glaucoma can be clinically evaluated using a variety of diagnostic tools but the following triad has been the cornerstone of diagnosis: [32]
-
Optic disc changes
-
Visual field changes
-
Elevated intraocular pressure
Optic Nerve
The optic nerve should ideally be evaluated using a slit lamp and 90D or 78D lens so that the 3-dimensional features of the optic nerve is better appreciated. Normally, the inferior neuroretinal rim (NRR) is the thickest, followed by superior, nasal, and temporal NRR. This is called ISNT rule. In OAG, this rule is not followed, as superior and inferior NRR gets thinned in the disease. The optic cup should be determined by its contour and not its color. A recent JAMA Rational Clinical Examination systematic review of primary open-angle glaucoma diagnosis found that the risk of glaucoma was highest when an examination revealed an increased cup-disk ratio (CDR), CDR asymmetry, disc hemorrhage, or elevated intraocular pressure.[33]
Typical optic nerve head changes in OAG include:
-
Diffuse or focal narrowing (notching/shelving) of the neuroretinal rim (NRR) specifically at the superior or inferior part of the optic disc
-
Symmetrically enlarged cup-to-disc ratio greater than 0.5
-
Increased vertical cup to disc ratio (CDR) and thinning of NRR
-
Asymmetry of CDR of 0.2 or more
-
Hemorrhage at or around the optic disc
-
Peripapillary atrophy
-
Baring of circumlinear vessels (gap between the superficial vessels and disc margin)
-
Bayonetting of vessels- The vessel first goes back and then climbs along the wall of the deep cup and then angles again on the disc margin
-
Very deep (excavated) cup with bean-pot cupping and laminar dot sign
-
Nasalization of optic disc vessels, and
-
Diffuse or focal (arcuate) thinning/defect of the retinal nerve fiber layer (RNFL) contiguous with an area of NRR-notch
-
The NRR is typically pink and not pale in OAG. Pallor of the NRR usually denotes an atrophic optic nerve as seen in primary angle closure glaucoma.
Visual Field
Perimetry, also known as visual field testing, is an important diagnostic tool that maps out the patient's visual field on a printout, making it a helpful and necessary tool in diagnosing and managing OAG. It is often helpful to get a baseline visual field for glaucoma suspects and confirmed OAG patients alike so that physicians can track the progression of the disease. To make a diagnosis of acquired glaucomatous visual field defect Hoddap–Parrish–Anderson criteria is used:[34]
-
Glaucoma hemifield test outside normal limits on at least 2 fields.
-
A cluster of three or more non-edge points in a location typical for glaucoma, all of which are depressed on the pattern deviation plot at a P <5% and one of which is depressed at a P <1% on 2 consecutive fields.
-
A corrected pattern standard deviation that occurs in less than 5% of normal fields on 2 consecutive fields.
Static automated threshold perimetry is used with white stimulus on a white background. Most studies used the Humphrey Field Analyzer, but other perimeters like Octopus have also been used successfully. SWAP (short-wavelength automated perimetry using blue stimulus on yellow background) and frequency doubling perimetry may pick up early visual field defects. The visual field must be reliable and field defects should be repeatable on at least 2 fields. The same machine, the same degree of field and protocol (eg, 24-2, 30-3, or 10-2) should be used to compare the fields to note for progression or stability. It has been estimated that at least 40%-50% ganglion cell loss is needed to reliably show visual field defects in threshold perimetry.[35][36] Thus, structural changes of the optic nerve occur earlier than functional change (visual field loss) in OAG. This gives rise to the concept of Preperimetric glaucoma which has been defined as 'the presence of characteristic glaucomatous changes in the optic disc and increased vulnerability to damage in the retinal nerve fiber layer (RNFL), without the presence of visual field defects detectable with standard automated perimetry'.[37]
Typical visual field changes in OAG include.
-
Increased variability of responses in an area which later developed field defects
-
Asymmetry of the visual field between the eyes
-
Paracentral scotoma- commonly superonasal
-
Roenne's nasal step- an area of depression above or below the horizontal meridian in the nasal side
-
Temporal wedge
-
Sickle-shaped (Seidel's) scotoma
-
Bjerrum's scotoma or arcuate scotoma
-
Annular/ring scotoma when arcuate scotoma is present on both above and below the horizontal meridian
-
General constriction of peripheral field
-
A temporal island of the visual field
Intraocular Pressure
When determining the IOP of a patient using tonometry, certain variables must be taken into consideration. Tonometry measurements can, for example, vary between examiners differing by approximately 10% per individual, which can translate to a difference in IOP measurement of 1 mmHg to 2 mmHg. An individual's corneal thickness or diurnal variations of IOP (e.g., higher IOP in early morning hours or other time of the day, or variability in the time of day of maximal IOP between patients) can also have a tremendous effect on the accuracy of IOP measurements. For this reason, multiple measurements should be taken in any patient suspected of having an elevated IOP, while also correlating measurements with both optic nerve and visual field examinations. If there are previous tonometry measurements available, they should be reviewed and compared to those that are most recent. Also, the IOP may be different at the same time of the day on different days. Different instruments may capture different values of IOP.
If a difference of 3 mmHg or more is noted between the two eyes, there should be an increased suspicion for the presence of glaucoma. Physicians should expect approximately 10% variation between individual measurements, and thus should repeat measurements over at least two to three occasions before deciding on the plan for treatment. Goldmann applanation tonometry (GAT) is thought as the gold standard for measuring IOP but is affected by corneal thickness. Higher corneal thickness gives falsely high values of IOP whereas low corneal thickness leads to a falsely low measurement of IOP.
Elevated intraocular pressure is an important and modifiable risk factor, however, it is not a diagnostic factor for OAG. An ophthalmologist should check the patient's intraocular pressure using applanation tonometry while remaining conscious of the fact that the nature of the applanation tonometry test causes patients to squeeze their eyes and artificially elevate their own pressure readings. Normal intraocular pressure should range between 12-21 mm Hg, however, approximately 2/3rds of patients with elevated intraocular pressure never develop glaucomatous optic nerve atrophy or visual field deficits. Once a patient has recorded a reliably high intraocular pressure reading above 21 mm Hg, they are deemed "glaucoma suspects." [19][38]
Gonioscopy
Open-angle glaucoma is a diagnosis of exclusion and other ocular emergencies such as closed-angle glaucoma must be ruled out immediately. Gonioscopy will essentially determine whether the diagnosis is considered "open" or "closed" angle glaucoma. Gonioscopy is an acquired skill that allows the ophthalmologist to visualize the angle between the cornea and iris and determine whether it is open. The angle between the iris and cornea should be 20-45 degrees to be considered "open" so that aqueous humor can circumvent from the posterior chamber to the anterior chamber.[19]
Optical Coherence Tomography
OCT is a diagnostic imaging modality that provides high-resolution cross-sectional imaging of the retina, optic nerve, and anterior segment. Low coherence infrared light is directed toward the back fo the eye and the path of scattered photons help recreate an image of the retina. OCT is highly reproducible and is thus widely used as an adjunct in routine glaucoma patient management. Peripapillary RNFL analysis would show thinning in this layer and is the most commonly used scanning protocol for glaucoma diagnosis because it samples RGCs from the entire retina. Some of the drawbacks included variability in ONH morphology from patient to patient. To overcome some of these disadvantages, the macular thickness has been proposed as a means of glaucoma detection given that50% of RGCs are found in the macula, and RGC bodies are thicker than their axons, thus are potentially easier to detect.[39]
Corneal Photokeratoscopy
Corneal photokeratoscopy, also known as corneal topography, has been shown to conIn patients with primary open-angle glaucoma, there is a forward shifting of the posterior and anterior corneal surfaces. This appears to be correlated with more advanced stages of functional damage, pointing to a possible link between corneal structural changes and duration and intensity of elevated intraocular pressure. Further studies are needed for this marker to be used in monitoring primary open-angle glaucoma patients.[29]
Treatment / Management
The goal of treatment of open-angle glaucoma is:
-
Prevention of progression of optic nerve head changes
-
Prevention of deterioration of visual field
To achieve this goal, the concept of Target IOP was introduced. It is the upper limit of IOP, below which it is estimated that the visual field and the optic nerve head/RNFL parameters will not deteriorate, and the quality of life of the patient will not get compromised.
Glaucoma medications:
Debate exists over the optimal time to initiate treatment of Open-angle glaucoma with some physicians initiating treatment of IOP once it reaches above only 21 mmHg, and others reserving treatment either until there is evidence of optic nerve damage or if the patient is at high risk of damage or progression of open-angle glaucoma.
Treatment should be initiated if signs of damage as a result of open-angle glaucoma are evident (e.g., disc hemorrhage, nerve fiber layer defects, asymmetric cupping, vertical ovalization or notching of the cup) or if symptoms of elevated IOP are present (e.g., halos, blurred vision, pain, IOP consistently above 28 mmHg to 30mmHg) due to the high risk of optic nerve damage in the setting of elevated IOP. Some physicians begin a monocular trial with medications only in one eye, to assess the effectiveness and side effects of chosen medications before treating both eyes. However, different eyes might have a different response to the same drug, asymmetric IOP fluctuation may occur, and the drug may have a contralateral effect.
A target IOP should be set individually depending on the severity of structural and functional damage, baseline IOP, age, race, family history, corneal thickness, corneal hysteresis, and other risk factors.[40]
Follow-up should also be scheduled based on the level of success in IOP reduction between visits (e.g., more frequent follow-up with slower progression in treatment response) and the severity of optic nerve damage/visual field loss.
Overall, treatment should be individualized, taking into consideration the risk factors, systemic complications of medication use, the patient's life expectancy, medical history, concomitant conditions, and the patient's desire to receive treatment. The target IOP should be revised based on the behavior of optic nerve head damage and visual function (visual field).
If IOP/visual field/optic nerve head worsens while the patient is on medical therapy the compliance to therapy must be checked. Also, systemic factors including diabetes, smoking, and nocturnal hypotension should be controlled. The physician should himself/herself confirm from the patient the drops and how many times the patient is using these. A check should also be done to note if the administration of topical drops is correct or not. Closure of eyelids after administration of drops with nasolacrimal duct occlusion may prevent systemic absorption of the topical medication.
Topical medications:
Prostaglandin analog- Reduces IOP by 25% -33%. The usual dose in once daily. Side effects include lengthening of eyelashes, pigmentation of lids/ iris, exacerbation of uveitis/herpetic infection, and cystoid macular edema. It is preferred as initial therapy.
-
Latanoprost
-
Travoprost
-
Bimatoprost
-
Tafluprost
-
Latanoprostene Bunod- This molecule donates has nitric oxide-donating property.
Adrenergic agents: Reduces IOP by 20%-25%. Brimonidine may cause allergic blepharoconjunctivitis and apnea/lethargy/bradycardia in children.
-
Brimonidine
-
Apraclonidine
Beta-blockers: Reduces IOP by 20%-25%. Non-selective beta-blockers should be avoided in chronic obstructive pulmonary disease and asthma. Other contraindications include heart block, hypotension, and bradycardia.
Carbonic anhydrase inhibitor: Reduces IOP by 15%-20%.[19]
-
Dorzolamide
-
Brinzolamide
Cholinergic/parasympathomimetic agents: Reduces IOP by 20%-25%.
-
Pilocarpine
Systemic agents:
These are used in the acute rise of IOP or when topical medications are not tolerated.
Carbonic anhydrase inhibitor:
-
Acetazolamide
Osmotic agents
-
Mannitol
-
Glycerol
Laser Therapy for OAG:
Laser trabeculoplasty
The indications of laser trabeculoplasty include
-
As a primary therapy for OAG
-
To reduce the number of glaucoma drops
-
Non-compliance/intolerance to medical therapy
-
Failure of medical therapy as a less invasive alternative therapy compared to surgery
The available methods of laser trabeculoplasty are:
-
ALT (Argon laser trabeculoplasty)- More than 75% of unoperated eyes have a good reduction of IOP initially. Within 5 years, 30% to >50% eyes need additional surgical management. Repeat ALT usually (90%) fails to control IOP by 2 years.
-
SLT (Selective laser trabeculoplasty)- It uses Q-switched frequency doubled Nd-YAG. The efficacy of SLT is similar to ALT.
-
Micropulse laser trabeculoplasty-
Diode Laser Cyclophotocoagulation (DLCP)
DLCP is a method for ablation of the ciliary processes which secrete aqueous. Indications for DLCP include:
-
Uncontrolled IOP in eyes with ambulatory vision if chances of surgical success are poor
-
Uncontrolled IOP in painful blind eyes or eyes with minimal visual potential
-
Uncontrolled IOP with maximal medication after failed glaucoma surgery/ies
Surgical Management of OAG:
Indications for surgical management of glaucoma are
-
IOP above target pressure or progression of visual fields and optic disc changes despite good compliance and maximally tolerable glaucoma medication
-
To avoid excessive glaucoma drops
-
Significant barriers to effective and regular medication use including cost, compliance, physical disability, inconvenience, side effects, psychosocial
-
Primary therapy for advanced glaucoma requiring very low target IOP
-
Patient preference over other options
Surgical options include
-
Trabeculectomy- Success rate may vary from 31-88%. The success rate increases with the use of mitomycin C or 5-fluorouracil. These agents, however, increase the risk of late-onset bleb leak, hypotony, and bleb-related infection.
-
Glaucoma drainage device- Molteno, Baerveldt, Ahmed.
-
Non-penetrating glaucoma surgery:
-
Deep sclerectomy
-
Viscocanalostomy
-
Canaloplasty
-
-
Minimally invasive or micro-invasive glaucoma surgery (MIGS)[41]- MIGS provides is a conjunctiva-sparing surgery with an ab-interno approach to reduce IOP in mild to moderate glaucoma. The different approaches include:[41]
-
'increasing trabecular outflow (Trabectome, iStent, Hydrus stent, gonioscopy-assisted transluminal trabeculotomy, excimer laser trabeculotomy);
-
suprachoroidal shunts (Cypass micro-stent);
-
reducing aqueous production (endocyclophotocoagulation); and
-
subconjunctival filtration (XEN gel stent)'
-
Cyclocryotherapy
This is another method of cycloablation using cryotherapy usually reserved for painfully blind eyes.
Painful blind eyes from glaucoma may need
-
Enucleation
-
Retrobulbar injection of absolute alcohol
Differential Diagnosis
-
Optic nerve head diseases including
-
Physiological cup- A deep cup with a healthy neuroretinal rim, normal retinal nerve fiber layer thickness, and no visual field defect. The disc size may be large
-
Optic disc drusen
-
Optic disc coloboma
-
Anomalous optic disc
-
Tilted disc
-
Ischemic optic neuropathy
-
-
Retinal diseases causing similar visual field defects
-
Branch retinal vein occlusion
-
Branch retinal artery occlusion
-
Retinitis pigmentosa
-
Panretinal photocoagulation
-
-
Central nervous system diseases
-
Pituitary tumor- The NRR is typically pale, pallor is more than cupping, and there is bitemporal hemianopia which respects vertical line passing through the fixation (contrary to glaucoma, it which visual field respects horizontal meridian)
-
Cerebrovascular accident
-
Multiple sclerosis
-
Staging
The Americal Academy of Ophthalmology (AAO) preferred practice pattern (PPP)[19] classifies the severity of glaucomatous damage to different categories:
Mild: definite optic disc or RNFL abnormalities consistent with glaucoma as detailed above and a normal visual field as tested with standard automated perimetry (SAP)
Moderate: definite optic disc or RNFL abnormalities consistent with glaucoma as detailed above, and visual field abnormalities in one hemifield that are not within 5 degrees of fixation as tested with SAP
Severe: definite optic disc or RNFL abnormalities consistent with glaucoma as detailed above, and visual field abnormalities in both hemifields and/or loss within 5 degrees of fixation in at least one hemifield as tested with SAP
Indeterminate: definite optic disc or RNFL abnormalities consistent with glaucoma as detailed above, inability of patient to perform visual field testing, unreliable/uninterpretable visual field test results, or visual fields not performed yet'
Prognosis
Advanced POAG may cause optic atrophy and no perception of light, though most OAG patients will not lose vision in their lifetime. Risk factors for progression of OAG include[19]:
-
Old age
-
Elevated IOP
-
Increased cup to disc ratio or small optic rim area
-
Beta peripapillary atrophy
-
Disc hemorrhage
-
Thin central corneal thickness
-
Reduced corneal hysteresis
-
Low ocular perfusion pressure
-
Poor compliance to therapy
-
Pseudoexfoliation
In 10 years, the cumulative probability of end-stage glaucoma in at least one eye in untreated cases was 35% in a study.[42]
Complications
Complications of glaucoma include:
-
Blindness- usually painless
-
Painful blind eye/Absolute glaucoma- OAG predisposes to central retinal venous occlusion, which can give rise to neovascular glaucoma and painful blind eye.
Consultations
Ophthalmology, comprehensive general
Ophthalmology, glaucoma specialist
Deterrence and Patient Education
Glaucoma is a preventable cause of blindness making patient education crucial in managing and preventing the progression of open-angle glaucoma. Effective and successful treatment open-angle glaucoma can prevent the evolution of optic nerve atrophy and preserve patients vision. However, patient adherence and compliance to medical therapy in patients with glaucoma is notoriously difficult. Treatment regimens require daily treatment to control intraocular pressure and this is can be a challenging task for patients for the rest of their life. The nature of medication regimens requiring daily dosage is difficult for many patients and inconsistent medication administration will not adequately control intraocular pressure. Some patients will attempt to use their drops every day but will fail to properly deliver the medications into their eye and thus the medication will not be absorbed, specifically at-risk elder populations, may struggle with administering drops into their own eyes. Over a prolonged time frame, failure to adhere to prescribed daily drops or oral medication will increase the likelihood of progression to blindness and thus produce a lower quality of life as well as increase overall downstream healthcare costs for the patient. Studies have confirmed an inverse relationship between the number and frequency of dosage and patient adherence with respect to glaucoma treatment. One study looked into the reasons why patients struggled to adhere to their regimens and the most commonly cited reasons were: forgetfulness (30%), other priorities (11%), lack of information (9%), emotional factors (7%) with 27% of individuals surveyed not providing a reason. Previous studies have shown that improved patient education regarding disease processes and the rationale behind treatment regimens makes patients consistently more likely to adhere to their prescribed medication regimens. One would think that a "lack of information" can be addressed by the physicians in the office but a 2018 study found that physician-centric multifaceted informational and educational mailings were not effective in improving adherence to IOP-lowering treatment in this population of elderly patients with glaucoma. Therefore, we know that patient education is an important factor in glaucoma management but we have yet to find the best way to address patient education. Interestingly, other studies have confirmed that there is no relationship between medication adherence and medication side effects, and that side effects are unlikely to deter patients. A deceiving component of glaucoma is that patients are usually asymptomatic and are not constantly being reminded of their disease process, patients do not sense their slow loss of vision until it is too late. This is where interprofessional healthcare teams can play an integral role in detecting patient with risk factors and symptoms in nursing homes and elderly care facilities. An interprofessional approach is necessary as not all patients see their ophthalmologist regularly thus making nurses, therapists, social care workers, and primary care physicians the first line of defense who can help these patients with risk factors get screened. [43][44][45][46][45][47][48]
Enhancing Healthcare Team Outcomes
If not treated, open-angle glaucoma (OAG) leads to progressive loss of peripheral vision followed by central visual field loss. Due to its chronicity, it is best managed by an interprofessional team to provide ongoing patient education and ensure that correct daily dosing is maintained. The best outcomes occur when the provider, ophthalmic specialty trained nurse, and pharmacist work together to provide ongoing education and support to the patient. [Level V]
Review Questions
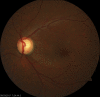
Figure
Glaucomatous optic nerve head with inferotemporal retinal nerve fiber layer defect. Contributed by Koushik Tripathy, MD
References
- 1.
-
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14;311(18):1901-11. [PMC free article: PMC4523637] [PubMed: 24825645]
- 2.
-
Liu K, He W, Zhao J, Zeng Y, Cheng H. Association of WDR36 polymorphisms with primary open angle glaucoma: A systematic review and meta-analysis. Medicine (Baltimore). 2017 Jun;96(26):e7291. [PMC free article: PMC5500050] [PubMed: 28658128]
- 3.
-
Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Héon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005 Mar 15;14(6):725-33. [PubMed: 15677485]
- 4.
-
Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010 Oct;42(10):906-9. [PMC free article: PMC3222888] [PubMed: 20835238]
- 5.
-
Borrás T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003 Jul;22(4):435-63. [PubMed: 12742391]
- 6.
-
Pasquale LR, Loomis SJ, Kang JH, Yaspan BL, Abdrabou W, Budenz DL, Chen TC, Delbono E, Friedman DS, Gaasterland D, Gaasterland T, Grosskreutz CL, Lee RK, Lichter PR, Liu Y, McCarty CA, Moroi SE, Olson LM, Realini T, Rhee DJ, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Allingham RR, Pericak-Vance MA, Weinreb RN, Zhang K, Hauser MA, Richards JE, Haines JL, Wiggs JL. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am J Ophthalmol. 2013 Feb;155(2):342-353.e5. [PMC free article: PMC3544983] [PubMed: 23111177]
- 7.
-
Nunes HF, Ananina G, Costa VP, Zanchin NIT, de Vasconcellos JPC, de Melo MB. Investigation of CAV1/CAV2 rs4236601 and CDKN2B-AS1 rs2157719 in primary open-angle glaucoma patients from Brazil. Ophthalmic Genet. 2018 Apr;39(2):194-199. [PubMed: 29111846]
- 8.
-
Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011 Jun;43(6):574-8. [PubMed: 21532571]
- 9.
-
Shim MS, Kim KY, Noh M, Ko JY, Ahn S, An MA, Iwata T, Perkins GA, Weinreb RN, Ju WK. Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line. Biochem Biophys Res Commun. 2018 Sep 18;503(4):2690-2697. [PMC free article: PMC6133749] [PubMed: 30100066]
- 10.
-
Ma L, Zeng Y, Wei J, Yang D, Ding G, Liu J, Shang J, Kang Y, Ji X. Knockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylation. Biomed Pharmacother. 2018 Nov;107:1728-1735. [PubMed: 30257391]
- 11.
-
Berner D, Zenkel M, Pasutto F, Hoja U, Liravi P, Gusek-Schneider GC, Kruse FE, Schödel J, Reis A, Schlötzer-Schrehardt U. Posttranscriptional Regulation of LOXL1 Expression Via Alternative Splicing and Nonsense-Mediated mRNA Decay as an Adaptive Stress Response. Invest Ophthalmol Vis Sci. 2017 Nov 01;58(13):5930-5940. [PubMed: 29164236]
- 12.
-
Guven Yilmaz S, Palamar M, Onay H, Ilim O, Aykut A, Ozkinay FF, Yagci A. Association of Lysyl Oxidase-like 1 Gene Polymorphism in Turkish Patients With Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. J Glaucoma. 2017 Jul;26(7):684-685. [PubMed: 28369001]
- 13.
-
Sharma S, Martin S, Sykes MJ, Dave A, Hewitt AW, Burdon KP, Ronci M, Voelcker NH, Craig JE. Biological effect of LOXL1 coding variants associated with pseudoexfoliation syndrome. Exp Eye Res. 2016 May;146:212-223. [PubMed: 26997634]
- 14.
-
Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology. 2014 Jan;121(1):134-141. [PMC free article: PMC4038428] [PubMed: 24823760]
- 15.
-
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006 Mar;90(3):262-7. [PMC free article: PMC1856963] [PubMed: 16488940]
- 16.
-
Distelhorst JS, Hughes GM. Open-angle glaucoma. Am Fam Physician. 2003 May 01;67(9):1937-44. [PubMed: 12751655]
- 17.
-
Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998 Dec;116(12):1640-5. [PubMed: 9869795]
- 18.
-
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B., BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008 Jan;115(1):85-93. [PubMed: 17629563]
- 19.
-
Prum BE, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, Herndon LW, Lim MC, Williams RD. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016 Jan;123(1):P41-P111. [PubMed: 26581556]
- 20.
-
Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007 Aug;454(5):849-55. [PubMed: 17351786]
- 21.
-
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006 Jul 20;497(3):326-49. [PMC free article: PMC2885916] [PubMed: 16736474]
- 22.
-
Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3460-6. [PubMed: 11006239]
- 23.
-
Morgan-Davies J, Taylor N, Hill AR, Aspinall P, O'Brien CJ, Azuara-Blanco A. Three dimensional analysis of the lamina cribrosa in glaucoma. Br J Ophthalmol. 2004 Oct;88(10):1299-304. [PMC free article: PMC1772339] [PubMed: 15377555]
- 24.
-
Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981 Jan;99(1):137-43. [PubMed: 7458737]
- 25.
-
Morgan-Davies J, King AJ, Aspinall P, O'Brien CJ. Measurement of a novel optic disc topographic parameter, "spikiness", in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2000 Aug;238(8):669-76. [PubMed: 11011687]
- 26.
-
Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994 Nov;101(11):1851-5. [PubMed: 7800368]
- 27.
-
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981 Apr;99(4):635-49. [PubMed: 6164357]
- 28.
-
Călugăru D, Mang A, Călugăru M. Secondary open-angle pigmentary glaucoma resulting from pseudophakia. Case report. Rom J Ophthalmol. 2016 Apr-Jun;60(2):125-130. [PMC free article: PMC5711363] [PubMed: 29450335]
- 29.
-
Gil P, Pires J, Matos R, Cardoso MS, Lopes N, Matias J, Mariano M. Corneal Elevation Topography in Primary Open Angle Glaucoma. J Glaucoma. 2017 Feb;26(2):e41-e45. [PubMed: 27599178]
- 30.
-
Alamri A, Alkatan H, Aljadaan I. Traumatic Ghost Cell Glaucoma with Successful Resolution of Corneal Blood Staining Following Pars Plana Vitrectomy. Middle East Afr J Ophthalmol. 2016 Jul-Sep;23(3):271-3. [PMC free article: PMC4968153] [PubMed: 27555716]
- 31.
-
McMenamin PG, Lee WR. Ultrastructural pathology of melanomalytic glaucoma. Br J Ophthalmol. 1986 Dec;70(12):895-906. [PMC free article: PMC1040858] [PubMed: 3801367]
- 32.
-
Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Semin Ophthalmol. 2017;32(2):237-250. [PubMed: 26148300]
- 33.
-
Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 2013 May 15;309(19):2035-42. [PubMed: 23677315]
- 34.
-
Susanna R, Vessani RM. Staging glaucoma patient: why and how? Open Ophthalmol J. 2009 Sep 17;3:59-64. [PMC free article: PMC2760888] [PubMed: 19834563]
- 35.
-
Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006 Jun;124(6):853-9. [PMC free article: PMC2265071] [PubMed: 16769839]
- 36.
-
Harwerth RS, Carter-Dawson L, Shen F, Smith EL, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999 Sep;40(10):2242-50. [PubMed: 10476789]
- 37.
-
Shiga Y, Aizawa N, Tsuda S, Yokoyama Y, Omodaka K, Kunikata H, Yasui T, Kato K, Kurashima H, Miyamoto E, Hashimoto M, Nakazawa T. Preperimetric Glaucoma Prospective Study (PPGPS): Predicting Visual Field Progression With Basal Optic Nerve Head Blood Flow in Normotensive PPG Eyes. Transl Vis Sci Technol. 2018 Jan;7(1):11. [PMC free article: PMC5782826] [PubMed: 29372113]
- 38.
-
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002 Feb;86(2):238-42. [PMC free article: PMC1771026] [PubMed: 11815354]
- 39.
-
Kansal V, Armstrong JJ, Pintwala R, Hutnik C. Optical coherence tomography for glaucoma diagnosis: An evidence based meta-analysis. PLoS One. 2018;13(1):e0190621. [PMC free article: PMC5754143] [PubMed: 29300765]
- 40.
-
Sit AJ, Pruet CM. Personalizing Intraocular Pressure: Target Intraocular Pressure in the Setting of 24-Hour Intraocular Pressure Monitoring. Asia Pac J Ophthalmol (Phila). 2016 Jan-Feb;5(1):17-22. [PubMed: 26886115]
- 41.
-
Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189-206. [PMC free article: PMC4734795] [PubMed: 26869753]
- 42.
-
Wilson MR. Progression of visual field loss in untreated glaucoma patients and suspects in St Lucia, West Indies. Trans Am Ophthalmol Soc. 2002;100:365-410. [PMC free article: PMC1358971] [PubMed: 12545702]
- 43.
-
Fiscella R, Caplan E, Kamble P, Bunniran S, Uribe C, Chandwani H. The Effect of an Educational Intervention on Adherence to Intraocular Pressure-Lowering Medications in a Large Cohort of Older Adults with Glaucoma. J Manag Care Spec Pharm. 2018 Dec;24(12):1284-1294. [PubMed: 29848186]
- 44.
-
Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011 Jan;59 Suppl:S93-6. [PMC free article: PMC3038505] [PubMed: 21150041]
- 45.
-
Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009 Nov;116(11 Suppl):S30-6. [PubMed: 19837258]
- 46.
-
Friedman DS, Quigley HA, Gelb L, Tan J, Margolis J, Shah SN, Kim EE, Zimmerman T, Hahn SR. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007 Nov;48(11):5052-7. [PubMed: 17962457]
- 47.
-
Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005 Jun;112(6):953-61. [PubMed: 15885795]
- 48.
-
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005 Aug 04;353(5):487-97. [PubMed: 16079372]
Source: https://www.ncbi.nlm.nih.gov/books/NBK441887/
0 Response to "Differential Analysis Continue Cups Alt 1 or Discontinue for the Month Ended May 31"
Publicar un comentario